In my last post, I showed the synthesis of Hexamminenickel (II)
Chloride. In this follow-up, I wish to show how we determined the
percentage of nickel in the resultant product via gravimentric
analysis using butandionedioxime (dimethyl gyoxime).
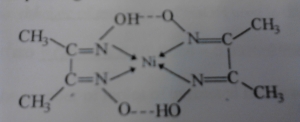
The reagent forms a red, square planar complex with Ni2+ in slightly
alkaline solution.
Structure of the complex:
A given mass of the “purple complex” (i.e. the Hexamminenickel (II)
Chloride) is dissolved in water, reacted with the butandionedioxime,
and the precipitate collected and weighed. By doing some additional
mathematics, we can use this to determine the amount of nickel in
the precipitate, and therefore, the amount in the “purple complex”.
Experimental:
- A sintered glass crucible (porosity 4) is cleaned, dried and its
mass taken to 4 decimal places. - 100ml of distilled water was poured into a 500ml beaker, and 5ml
of 6M HCl was added. - 0.2993g of Hexamminenickel (II) Chloride was added to the HCl
solution with rinsings, and the solution diluted to ~200ml with more
distilled water. - The solution was stirred to dissolve the Hexamminenickel (II) Chloride,
which dissolved readily. The solution was then heated to 75*C and 35ml
butandionedioxime added with stirring. - Approx 15ml or so of concentrated NH3 solution (aqueous) was added to
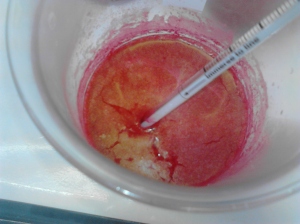
the solution with stirring. A further ~20 drops were added once a permanent red precipitate was obtained. - The solution was re-heated to 75*C and left for 25 minutes to permit the
precipitate to coagulate. - A drop of 2M NH3 soln. was injected into the clear part of the soln. to
test if further precipitation would occur. No further precipitation was
observed, so the precipitate was left to stand at room temp for 45 minutes. - The precipitate was filtered using the crucible previously dried and weighed under vacuum, and washed with 100ml of distilled water.
- The crucible was placed in an oven and left there overnight as I realized on checking after an hour that the precipitate was not yet fully dried, hence, I decided to come back the next day upon realizing the “wet” measurements had completely screwed up my calculations.
- The crucible, when dry completely, was weighed to 4 decimal places on an analytical balance (the kind dealers wish they had!).
Results:
Mass of [Ni(NH3)6]Cl2 weighed out: 0.2993g
Dry mass of sintered glass crucible: 10.3840g
Mass of the crucible + red ppt: 10.7336g
Mass of the red precipitate: 0.3496g
Molecular formula of red ppt: NiN4C8H14O4
Molecular mass of red ppt: 288.917g mol-1
%Ni in the red precipitate: 20.314%
Mass of Nickel in red ppt: 0.071g
%Nickel in the [Ni(NH3)6]Cl2 complex: 23.72%
Full Disclosure regarding the writeups: I will be borrowing heavily from the
laboratory notes and lecture notes of my college and lecturers (who will be
un-named for security and privacy reasons) while writing up the experiments I perform in said institute on this blog. Most of the reason I actually do these writeups is as a study aid for myself, and also because I like having an excuse to take photographs during labs. I take no credit for the originality of the work I present here, as most of it would not happen without the help of the excellent teaching staff. I would actually simply post OCR’d photocopies of my lab notebook with additional photographs, but I suspect that would be overstepping some kind of ethical line, so instead I do my best to reword things in an understandable (to myself) fashion and describe how things actually were done in the lab. Sometimes though, the description from notes simply is the best one there is.
If any of my lecturers happen to stumble across this, thanks for tolerating
the not-so-stealthy taking photos of chemicals and suchlike 😉
Finally, the most excellent Taylor Swift/Breaking Bad parody: